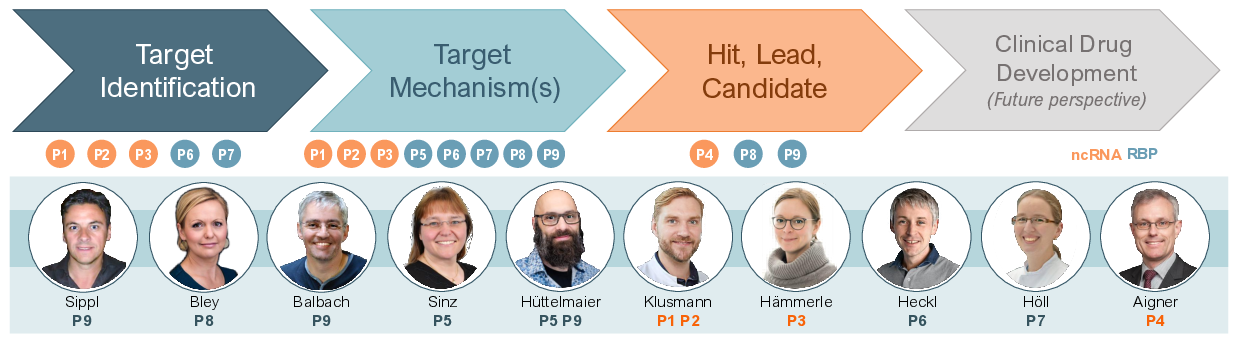
The RU5433 encompasses nine research projects (P01-P09), which aim to: (1) identify candidate targets of R3oGE in cancer, (2) elucidate the underlying target mechanisms in malignancies, and (3) initiate the pre-clinical development therapeutics for target inhibition in cancer therapy.
| NO. | Project Title | Project Leader |
|---|---|---|
| P01 | Deciphering the genetically interactive network of the DLK1-DIO3 ncRNA locus in the hematopoietic system and in infant leukemias | Klusmann (Frankfurt) |
| P02 | Targeting the non-coding stem cell signature in childhood acute myeloid leukemia | Klusmann (Frankfurt) |
| P03 | Deciphering the role of LINC00261 and FOXA2 as transcriptional regulators shaping the expression landscape of pancreatic cancer | Hämmerle (Halle) |
| P04 | Development of novel nanoparticle formulations for therapeutic in vivo lncRNA knockdown and circRNA-mediated oncomiR inhibition | Aigner (Leipzig) |
| P05 | The role and target potential of the RNA-binding protein MEX3A in lung adenocarcinoma (LUAD) | Hüttelmaier/Sinz (Halle) |
| P06 | Discovery and characterization of EZH2-regulated RBP feed-forward mechanisms controlling cellular transformation | Heckl (Halle) |
| P07 | Deciphering RBP networks in high-risk subgroups of pediatric ALL and AML | Höll (Halle) |
| P08 | The role and target potential of IGF2BP1 in ovarian cancer progression and immune evasion | Bley (Halle) |
| P09 | Targeting oncofetal RBPs by small molecule drugs in cancer | Balbach/Hüttelmaier/Sippl (Halle) |
| P10 | Coordination and RIF-associated Research Training Academy (RIF-RTA) | Hüttelmaier/Klusmann (Halle/Frankfurt) |
Focuses on acute megakaryoblastic leukemia (AMKL) that occurs most frequently in infants, has strong developmental ties, and is associated with poor prognosis. Previous DFG-funded (KL 2374/2-1; Emmy Noether-Program) analyses using a ncRNA expression atlas of the human hematopoietic system (www.lncScape.de) deciphered coordinated expression of the DLK1-DIO3 locus – which contains the largest miRNA cluster of the human genome, numerous box C/D snoRNAs and lncRNAs – in megakaryocytes and AMKL. As a part of the DFG-funded project (KL 2374/3-1), the investigators laid a foundation for interrogating the role of the DLK1-DIO3 locus in megakaryopoiesis and AMKL pathogenesis. For instance, those miRNAs within the locus were identified that drive megakaryocytic differentiation, as well as a novel cis-regulatory lincRNA (LINC02285) that affects megakaryopoiesis and controls expression of the locus. The proposed follow-up project for the RU5433 aims to further delineate the interacting genetic network of the DLK1-DIO3 polycistronic locus and its ncRNA-dependent regulation of normal and malignant hematopoiesis. This will be achieved by investigating individual miRNAs and lncRNAs of the DLK1-DIO3 locus in vivo using an established AML xenobank and xenotransplantation system (ERC starting grant agreement No. 714226) applying MISTRG mice, which express human cytokines (e.g. M-CSF) from their corresponding murine endogenous loci. Combinatorial effects of ncRNAs in the DLK1-DIO3 locus will be assessed by utilizing cell lines allowing the deletion of specific cis-elements via loxP sites inserted by CRISPR-Cas9 technologies (with P06). The functions and mechanisms of the ncRNAs will be resolved by molecular characterization of lncRNAs (with P03 and P05), identification of miRNA target genes and elucidating the function of box C/D snoRNAs in AMKL. The therapeutic potential of ncRNAs will be explored by pre-clinical testing of RNA therapeutics delivered using the established lipid nanoparticle (LNP)-packaging platform (with P02) and in collaboration with P04. Common platforms established by this group for patient-derived xenografts (PDX) and comprehensively characterized patient cohorts will be made available for other leukemia-related projects within the RU5433 (P02, P06 and P07).
Aims to dissect an lncRNA stem cell signature that is shared between pediatric AML blasts and normal hematopoietic stem cells (HSCs). This signature was discovered by comprehensively profiling the ncRNA landscape of the human hematopoietic system and supported by the DFG (www.lncScape.de; KL 2374/2-1). The working hypothesis of P02 is that lncRNAs from the core stem cell signature shape the cellular chromatin and transcriptional landscape. Thereby, they coordinate self-renewal, proliferation and differentiation in normal and malignant stem cells. Understanding such ncRNA-mediated mechanisms and their hijacking in malignant transformation will lay a foundation for developing novel targeted cancer-specific therapies. To identify functionally relevant stem cell-specific lncRNAs, two complementary CRISPRi-based screening strategies were applied in vitro and in vivo. Importantly, knockdown of 17 lncRNA genes, e.g. LINC00982, efficiently perturbed leukemic growth. LINC00982 is highly expressed in HSCs, maintained in leukemic blasts (bulk), particularly those with FLT3-ITD, and high expression of this lincRNA is associated with poor survival in the NCI-TARGET pediatric AML cohort. In this project the applicants will therefore characterize 5 high confidence stem cell-specific lncRNAs in depth (incl. LINC00982) that are essential for leukemia progression and will resolve their functional and mechanistic features by: CRISPR-Cas9-based or Cre/lox-P recombinase-mediated excision (with P06), deciphering their impact on normal hematopoiesis in vitro and in vivo (with P06 and P07), and delineating partners and targets within the oncogenic protein-interactome (with P03 and P05). Pre-clinical therapeutic interventions will be evaluated in PDX models using the established LNP packaging and delivery platform (see P01) and in collaboration with P04. The proposed project is supported by resources derived from and maintained by an ERC-funded project (ERC starting grant agreement No. 714226).
Focuses on the role of lncRNAs in pancreatic ductal adenocarcinoma (PDAC), a malignancy with a 5-year survival rate of less than 10%. The investigation of deregulated lncRNA expression in publicly available PDAC transcriptome data in collaboration with the coordinators of NGS analyses (Glaß and Misiak) revealed that the downregulation of LINC00261 in PDAC is associated with reduced overall survival probability and enhanced EMT-like (epithelial-to-mesenchymal transition) gene expression. These studies suggest that LINC00261 impairs EMT-like progression of PDAC in a partially TGFB-dependent manner by interacting with FOXA2, a crucial regulator of endoderm differentiation and gene locus adjacent to LINC00261. Studies proposed by P03 aim at deciphering mechanisms underlying TGFB/LINC00261/FOXA2-dependent deregulation of R3oGE in PDAC progression and will reveal the role and regulatory principles of lncRNAs, using CRISPR technologies and lncRNA-centered interaction studies (with P01-P02 and P05-P06), modulating the gene expression landscape in PDAC. Notably, the work program will concomitantly address a putative RBP-role of FOXA2 (with P05, P07 and P08). The ultimate aim of P03 is to reveal therapeutic vulnerabilities, manifested by deregulated lncRNA/FOXA2-dependent control of the transcriptional landscape in PDAC (with the coordinator of NGS analyses). The applicant’s expertise will support the pathological assessment of experimental tumor models, including the investigation of immune cell infiltration (P08), pathological evaluation of LUAD transgene mouse models (P05) and provides support in evaluating organ toxicity of therapeutics (P04 and P09).
Focuses on the development, refinement and application of novel nanoparticles for the delivery of small RNA molecules as therapeutics. Several lncRNAs and miRNAs are overexpressed in tumors and have been validated as potent tumor-promoting ncRNAs, thus representing attractive potential targets. Their inhibition by depleting or decoying RNA therapeutics, e.g. siRNAs or GapmeRs for lncRNA knockdown or circular RNAs as miRNA decoys, is feasible. However, these strategies critically rely on the efficient delivery of such RNA therapeutics. In previous studies, P04 has already established and tested nanoparticle delivery platforms for small RNAs. Recent work in collaboration with the Hüttelmaier (P05 and P09) lab also revealed the suitability of nanoparticle delivered circular miR-21-5p RNA decoys for impairing tumor growth in experimental LUAD animal models. Settling on this expertise, P04 will establish and explore novel chemically modified polyethylenimine (PEI)-based nanoparticle formulations, as well as combined formulations with liposomes (i.e., lipopolyplexes) for the in vitro, ex vivo and in vivo delivery of aforementioned RNA therapeutics. These formulations will be tested in various in vitro and ex vivo models as well as in vivo in colorectal and gastric carcinoma cell line-derived xenograft and in xenopatient (AVATAR) models. Studies on circular RNA decoys will be pursued in close collaboration with P05, providing circular miRNA decoys (ciRs) suitable for the inhibition of tumor-promoting miRNAs in cellulo and invivo. The delivery of RNA therapeutics by established and/or improved nanoparticle formulations to test the target potential of ncRNAs and RBPs will be explored in own models/targets as well as collaboration with projects characterizing the role of ncRNAs (P01, P02) or RBPs (P06, P07).
Will address the role and therapeutic target potential of the RBP MEX3A in lung adenocarcinoma (LUAD), which still accounts for the vast majority of cancer-associated mortality worldwide. MEX3A is a bona fide oncofetal RBP with reported E3-ubiqutin ligase activity. Unpublished findings by the applicants indicate that MEX3A promotes tumor growth in LUAD cell and animal models by enhancing major oncogenic drivers like MYC and impairing tumor suppressive pathways and regulators, e.g. TP53 and PDCD4. Therapeutic target potential of MEX3A was recently validated with P04 by revealing impaired tumor growth upon MEX3A depletion in experimental LUAD xenograft models. With the studies proposed, P05 will characterize the mechanisms underlying the presumably interconnected roles of MEX3A in controlling RNA fate (RNA-binding) and ubiquitin-dependent modulation (E3-ligase) of protein function and turnover. These efforts benefit from long standing collaboration and complementary expertise of the applicants: Hüttelmaier, the role and therapeutic target potential of ncRNAs and RBPs in cancer; Sinz, analyses of protein abundance, modification and ligand-association (incl. RBPs) by quantitative and cross-linking mass spectrometry. Investigation of MEX3A’s role in cellulo, xenograft and established transgenic LSL-MEX3A (LSL: lox-stop-lox) mouse tumor modelswill be pursued in close collaborationwith P03 (pathological evaluation) and P08 (cell phenotyping and immune evasion). The feasibility of directly inhibiting MEX3A will be explored by the SAR-guided development of small molecule inhibitors with P09. Expertise of both applicants supports mass spectrometry analyses within the RU5433 (P01-P04 and P06-P09) and the analysis of syngeneic and xenotransplant ovarian cancer models (P08).
Will investigate the role of RBPs in the EZH2-dependent regulation of gene expression in AML. Recently, P06 revealed that the transformation of hematopoietic progenitor cells upon EZH2 loss is associated with the severe upregulation of various RBPs, including the oncofetal IGF2BP3 (also see P08 and P09). Expression of some of these RBPs mimicked the EZH2 loss in bone marrow transplantation (BMT) models. This suggests that EZH2-regulated RBPs are essential drivers of transformation in AML. Aiming to reveal RBP-linked therapeutic vulnerabilities, P06 will identify RBPs involved in EZH2-dependent transformation by oncogene replacement (gain-of-function) and loss-of-function studies using CRISPR/Cas9 technologies. Complemented by functional genomic studies and the investigation of mechanisms as well as effector pathways underlying RBP-driven transformation (with P01-P02, P05 and P07), P06 will set the stage to explore the therapeutic target potential of identified RBPs and RBP-linked vulnerabilities. This will pave the way for proceeding with the pre-clinical testing of therapeutic strategies aiming at the depletion of RBPs by RNA-therapeutics (with P01-P02 and P04) and/or their inhibition by small molecules (with P09). Thus, the proposed studies link investigation of pathophysiological mechanisms of RBP-driven transformation in AML with therapeutic target validation to ultimately pursue the development of therapeutic strategies in leukemia treatment.
Pediatric Oncology Center
Will focus on RBP-mediated gene regulation in high-risk AML and ALL. Understanding, which RBPs are oncogenic drivers (oncoRBPs) and the identification of their main effector (m)RNAs will allow for the identification of therapeutic vulnerabilities by revealing new targetable proteins or pathways. To delineate the role of oncoRBPs in AML and ALL systematically, P07 (in collaboration with P06) has performed RBP-directed CRISPR/Cas9 screens in four AML and ALL cell lines. The aim of the proposed studies thus is to further explore their pathophysiological role and therapeutic target potential. To this end, P07 will validate oncogenic functions for prime candidates identified by CRISPR/Cas9 screening (with P01-P02 and P06). Through gain- and loss-of-function studies in human HSPCs in vitro, and in murine HSPCs as well as patient-derived xenografts in vivo, oncoRBP function in both, normal hematopoiesis and leukemogenesis, will be investigated (with P01-P02 and P06). Key effector (m)RNAs and pathways of RBPs will be identified by PAR-CLIP to identify oncoRBP-associated transcripts and elucidating deregulated gene expression by RNAseq upon perturbing oncoRBP expression (with P05-P06andP08). Hematopoietic differentiation in gain- and loss-of-function experiments will be explored in collaboration with P01-P02 and P06. Platforms for PDX models and characterized patient cohorts will be obtained from P02 and P06. The pre-clinical evaluation of targeting oncoRBPs (future perspective) will be pursued in collaboration with P09 (small molecule inhibitors) and P02 as well as P04 (RNA therapeutics).
Aims at deciphering mechanisms and target potential of the oncoRBP IGF2BP1 (main objective) and recently identified candidate oncoRBPs (incl. MEX3A studied by P05) in HGSC (high-grade serous ovarian cancer), which has an age-associated 5-year survival rate below 30-50%. The analysis of HGSC transcriptomes in collaboration with the coordinators of NGS analyses (Glaß and Misiak) revealed that IGF2BP1 de novo synthesis is primarily observed in the mesenchymal-like and immune-suppressed molecular C5 subtype of HGSC. In agreement, P08 (with P05) previously demonstrated that IGF2BP1 is a post-transcriptional enhancer of mesenchymal tumor cell properties, tumor growth, peritoneal spread and metastasis in experimental HGSC models. Recent investigations of these models, supported by the RTG1591, further revealed that IGF2BP1 promotes immune evasion and influences the DNA damage response (DDR) in ovarian cancer cells. Studies proposed by P08 aim at characterizing the mechanisms underlying these tumorigenesis-promoting roles of IGF2BP1 in HGSCs and will identify key downstream effectors in experimental human and murine ovarian cancer cell models. Findings will be further explored in murine and patient-derived organoids (with P03and P05) investigated in collaboration with the MLU’s clinic of gynecology. These efforts set the stage to address the target potential of IGF2BP1 in HGSC therapy. To this end, P08 will evaluate therapeutic synergies of direct IGF2BP1 inhibition (IGF2BP1i; with P05 and P09) with treatment concepts aiming to impair immune evasion, specifically by targeting PD-L1/PD1, and PARP inhibition (PARPi). Immune cell infiltration in experimental and clinical tumor samples will be analyzed by multi-spectral imaging (MSI) with P03. Combinatorial inhibition of IGF2BP1 and PARP as well as other candidate therapeutics benefits from close interactions with the MLU’s clinics of gynecology. The ample expertise of the applicant in cell phenotyping, including live cell and high resolution imaging (CFI, Core Facility Imaging) will support studies proposed by P01-P03, P05-P07 and P09.
Aims at the SAR-guided development of RBP-directed small molecule inhibitors for cancer therapy. In previous studies, IGF2 mRNA binding proteins (IGF2BPs) were demonstrated to promote tumor progression in a conserved manner. IGF2BP1, the most potent oncofetal RBP of the IGF2BP family, impairs the mostly miRNA- and m6A-dependent (N6-methyladenosine) inhibition of tumor promoting factors, such as E2F1, LIN28B, MYC/N and SRF. This post-transcriptional enhancement of pro-oncogenic factors is effectively inhibited in cells and animal tumor models by reversible as well as covalent cysteine-directed small molecules interfering with IGF2BP1-RNA association (patents: EP20159945.3, EP202118071.1). Based on the complementary expertise in structural biology (Balbach), RBP-centered R3oGE in tumor biology (Hüttelmaier) and medicinal chemistry (Sippl), P09 will pursue the SAR-guided development and refinement of IGF2BP1/3-directed (also see P06 and P08) reversible, irreversible (covalent) inhibitors as well as PROTACs (proteolysis targeting chimeras) and will initiate the development of covalent inhibitors directed against other oncofetal RBPs like MEX3A (also see P05). These efforts provide a platform and technologies for exploring small molecule inhibition of other oncoRBPs characterized by the consortium, in particular P06-P08. Studies proposed by P09 require support in investigating protein modification by mass spectrometry (with P05) and the evaluation of small molecule-associated toxicity associated in animal tumor models (with P03) and primary human cells (with P08). The potency of developed RBP-inhibitors in leukemic (with P01-P02 and P06-P07), pancreatic (with P03) cancer models as well as immune evasion (with P08) will be explored.
Aims to provide an RTG-like infrastructure, termed the RNA in Focus Research Training Academy (RIF-RTA).

