Research Program
Pancreatic ductal adenocarcinoma (PDAC) is a particularly aggressive cancer with a very poor prognosis. The 5-year survival rate for PDAC is only about 8 %, and the majority of patients are diagnosed at a late stage of the disease. This is in part due to the lack of reliable biomarkers for early detection of PDAC.
Chronic inflammation has been shown to play a role in the development of many cancer types, including PDAC. However, the specific molecular mechanisms by which inflammatory cues trigger early pancreatic carcinogenesis are not well understood.
This RTG aims to comprehensively understand these molecular mechanisms to improve the chances of early diagnosis and detect novel treatment avenues for PDAC. It is a collaborative effort of clinical scientists with expertise in various aspects of pancreatic research and specialties such as gastroenterology, visceral surgery, and oncology, as well as basic researchers with expertise in molecular biology, anatomy, pharmacology, and protein mass spectrometry. The team also includes early career researchers with expertise in cutting-edge technologies such as functional genomics, epigenetics, bioinformatics, and molecular pathology. This offers a unique setting to elucidate the complex mechanisms of inflammation-induced early pancreatic carcinogenesis.
Main research areas
The RTG is divided into three main research areas, under which the individual research projects are organized (A: In vivo modeling, B: In vitro modeling, C: Target characterization).
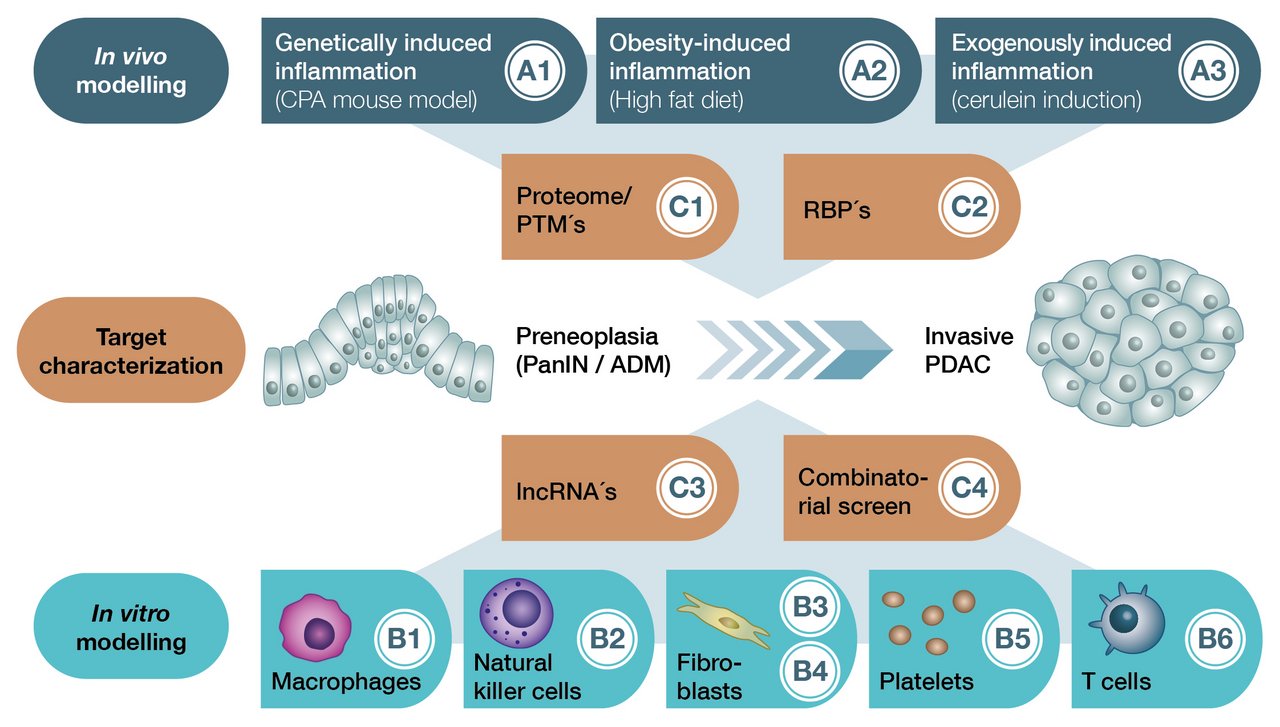
Projects A1 - A3 seek to comparatively characterize three different mouse models (in vivo = in the living organism). These replicate genetically (carboxypeptidase A mutation), obesity (high fat diet) and exogenously (caerulein) induced chronic inflammation of the pancreas. Herein, the effects on early K-Ras-dependent carcinogenesis are studied.
Projects B1 - B6 use different in vitro modeling approaches (in vitro = outside the organism) based on the mouse models of projects A1 - A3. The aim here is to decipher the effects of different stromal components such as macrophages, NK cells, fibroblasts, platelets and T cells on malignant transformation.
Projects C1 - C4 aim to functionally characterize candidate genes that are differentially regulated during inflammation-associated carcinogenesis. This will help to identify novel diagnostic and therapeutic targets.

