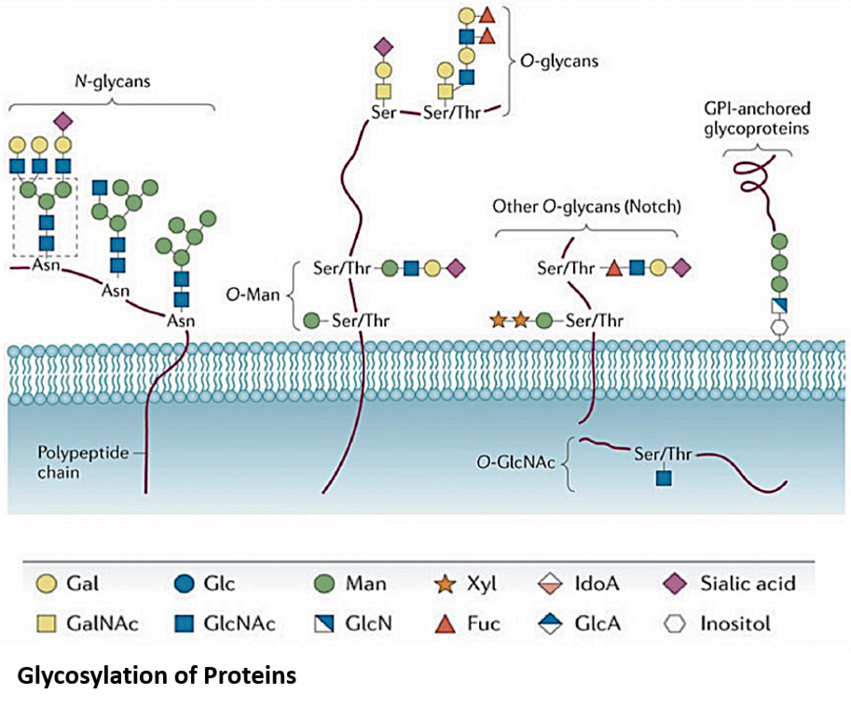
The focus of our research is on sugar (= glycan) structures attached to proteins. Such glycan structures consist of several sugars (monosaccharides) as building blocks. This so-called glycosylation of proteins is very complex and modulates folding, localization or half-life of the respective proteins and thereby influences their function in general. Nearly 50% of all proteins are glycoproteins. Although glycosylation of proteins occurs at specific sequences, glycosylation is not encoded in the gene of the respective protein but is regulated by the presence of specific enzymes or substrates. Therefore, a given protein can exist in hundreds of glycoforms and glycosylation thereby increases the diversity of proteins by several orders of magnitude. Defects in the glycosylation of proteins (congenital disorders of glycosylation = CDG) result in various defects and the affected patients suffer on serious, sometimes fatal, disfunction of several different organ systems.
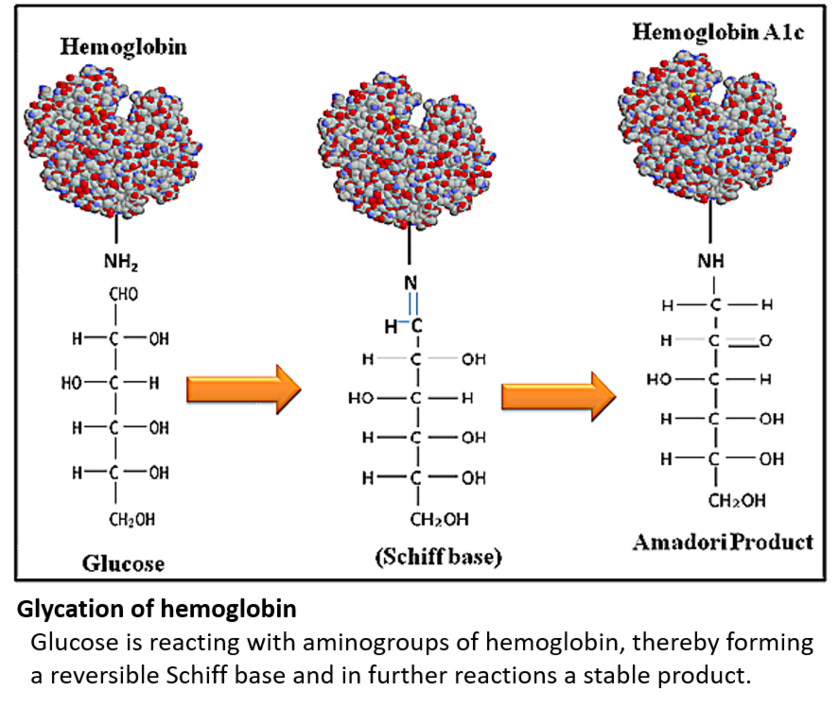
Furthermore, proteins can react with carbonyl groups (for example of monosaccharides) in a chemical reaction called glycation. This reaction is not catalyzed by enzymes and results in the formation of advanced glycation end products (AGEs). Glycation of proteins increases with age and often leads to malfunction of the glycated proteins. It is generally accepted that glycation is implicated in many age-related chronic diseases.
Both reactions (glycosylation = enzymatic and glycation = non-enzymatic) have in common that they form posttranslational modifications (PTMs) on the respective proteins.
We are studying posttranslational modifications in the context of ageing and tumor progression. Thereby we interfere with posttranslational modifications in cell cultures and analyze biological readouts such as enzymatic activity, cell-adhesion, cell-migration or cell-invasion.
The UDP-N-acetylglucosamine-2-epimerase / N-acetylmannosamine kinase (GNE) catalyzes the first two steps of the sialic acid, the irreversible epimerization from UDP-GlcNAc to ManNAc and its subsequent phosphorylation to ManNAc6P. Both enzyme activities are located on one polypeptide, therefore GNE represents a bifunctional enzyme. The epimerase domain is N-terminal located and the kinase domain C-terminal. The epimerase activity of GNE underlies a strong feedback inhibition by CMP-Neuraminic 5-acid (Neu5Ac), the endproduct of the metabolic pathway. GNE epimerase activity is regulated by self-associated oligomeric states of the GNE protein. GNE exists as different oligomers, homodimers, -tetramers or – hexamers]. The hexamer/tetramer exhibits epimerase- as well as kinase activity, whereas the dimer exhibits only kinase activity. Protein kinase C mediated phosphorylation of GNE leading to an increased GNE epimerase activity.
154 mutations in the gene of GNE have been identified so far, likely leading to reduced GNE enzymatic activities. Mutations in the GNE sequence are involved in the development of the neuromuscular disorder GNE myopathy (formerly hereditary inclusion body myopathy, HIBM), which accompany with a progressive amyotrophia during ageing. Prominently, proximal and distal skeletal muscles are affected. After two or three progressive decades, the patients are tightly restricted in their mobility and are wheelchair-dependent. Previously, 122 of reported mutations so far, which could lead to a phenotype of GNE myopathy, are missense mutations. ~23% of these mutations, will be substituted in the protein sequence to threonine (T) or serine (S). The distinctive features of some mutations is the introduction of novel potential phosphorylation or rather GlcNAcylation sites. O-linked β-N-acetylglucosamine transferase (O-GlcNAc-transferase, OGT) catalyzes the transfer of N-acetylglucosamine (GlcNAc) via an O-glycosidic linkage to a serine or threonine residues of intracellular proteins. O-Phosphate and O-GlcNAc are known to compete for the hydroxyl group of threonine or serine residues. Phosphorylation or O-GlcNAcylation can occur on the same protein close together. In addition, phosphorylation and O-GlcNAcylation are dynamic PTMs and therefore exchangeable within a protein.
Glycation is a process in which carbonyls, such as sugar molecules like glucose or fructose, react with amine residues of proteins or lipids without involvement of enzymes. Following further chemical, non-enzymatic reactions, so-called advanced glycation end products (AGEs) are formed, which lead to protein crosslinking and dysfunction. Not only sugars, but also other physiologically occurring metabolites are able to form AGEs. Highly reactive dicarbonyl metabolites can lead to glycation and are produced in the human body during lipid peroxidation, amino acid oxidation and as side products of metabolic pathways like glycolysis. Dicarbonyl metabolites are the most important intermediates in non-enzymatic protein modification and are much stronger glycating agents then sugars. Two prominent dicarbonyls existing in mammals are methylglyoxal (MGO) and glyoxal (GO). An increase in the steady state level of reactive carbonyl species is defined as carbonyl stress. Under physiological conditions dicarbonyls are detoxified via the glyoxalase system, which prevents AGE formation. During aging the glyoxalase activity is reduced. Aging is associated with an accumulation of AGEs which are thought to be responsible for the pathogenesis of cardiovascular and metabolic diseases and impairments. Supporting this idea is the observation that high levels of dicarbonyls can be found in the plasma of patients with age-related diseases like type II diabetes and obesity.
Sialic acid (Sia), which is expressed as the terminal sugar in many glycoconjugates, plays an important role during development and regeneration. Addition of homopolymers of Sia (polysialic acid = polySia) to the neural cell adhesion molecule (NCAM) is a unique and highly regulated posttranslational modification. The presence of polySia affects NCAM-dependent cell adhesion and plays an important role during brain development, neural regeneration and plastic processes including learning and memory. Polysialylated NCAM is expressed on several highly malignant neuroendocrine tumors, and its presence correlates with poor prognosis. Two closely related polysialyltransferases, ST8SiaII and ST8SiaIV, catalyze the biosynthesis of polySia. PolySia expression is high during embryogenesis, peaks in the perinatal phase and decreases rapidly in the adult, where only plastic regions in the brain remain polySia positive. However, in adults it is still present in some neuronal regions such as the olfactory bulb, hippocampus and hypothalamus. Enzymatic removal of polySia using endoneuraminidase (endo-N) induces neurological abnormalities similar to those in NCAM-deficient mice. These abnormalities include deficits in hippocampal long-term potentiation (LPT) and long-term depression (LTD), spatial learning, cell migration and axonal targeting. Polysialylated NCAM has been linked to the malignant potential of several tumors: alveolar rhabdomyosarcoma (RMS), undifferentiated neuroblastoma, anaplastic Wilms tumor, aggressive colon cancers), pancreatic cancer and all neuroendocrine lung tumors.
N-Acetylneuraminic acid is the most prominent sialic acid in eukaryotes. The structural diversity of sialic acid is exploited by viruses, bacteria and toxins, and by the sialoglycoproteins and sialoglycolipids involved in cell-cell recognition in their highly specific recognition and binding to cellular receptors. The physiological precursor of all sialic acids is N-acetyl D-mannosamine (ManNAc). By recent findings it could be shown that synthetic N-acyl-modified D-mannosamines can be taken up by cells and efficiently metabolized to the respective N-acyl-modified neuraminic acids in vitro and in vivo. Successfully employed D-mannosamines with modified N-acyl side chains include N-propanoyl- (ManNProp), N-butanoyl- (ManNBut)-, N-pentanoyl- (ManNPent), N-hexanoyl- (ManNHex), N-crotonoyl- (ManNCrot), N-levulinoyl- (ManNLev), N-glycolyl- (ManNGc), and N-azidoacetyl D-mannosamine (ManNAc-azido). All these compounds are metabolized by the promiscuous sialic acid biosynthetic pathway and are incorporated into cell surface sialoglycoconjugates replacing in a cell type-specific manner 10 to 85% of normal sialic acids. Application of these compounds to different biological systems has revealed important and unexpected functions of the N-acyl side chain of sialic acids.
We are happy to welcome interested students in the lab. There lots of possibilities, including internships, Bachelor or Master thesis and doctoral thesis (medicine or natural science). Please feel free to contact us.



